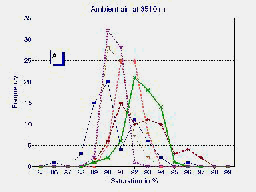
 A
A
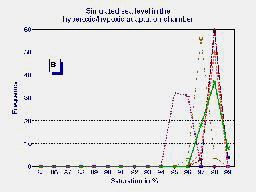 B
B
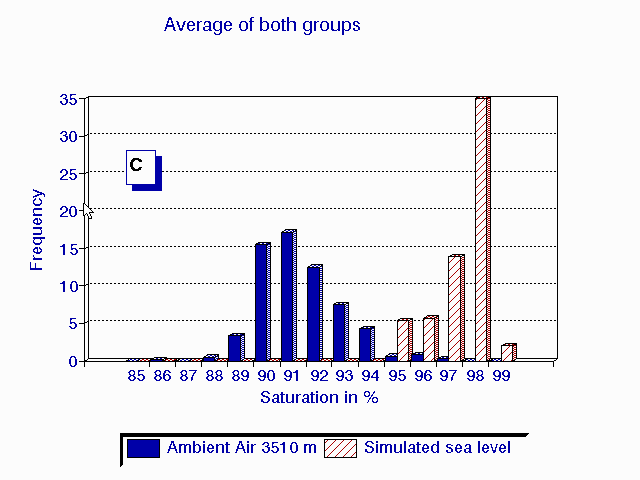 C
C
HIGH ALTITUDE PATHOLOGY INSTITUTE (IPPA).
E-mail: zubieta@altitudeclinic.com
P.O. Box 2852
La Paz, Bolivia
SUMMARY
With the advent of pulse oximetry, oxygen saturation measurements have
been simplifiehttp://www.altitudeclinic.com/images/graphcc1.gif
http://www.altitudeclinic.com/images/graphcc1.gifd. However, at high altitude, large fluctuations from breath
to breath have been observed.
Hence, in order to evaluate the performance of pulse oximetry, 20 normal
native residents of the bowl-shaped city of La Paz (3100-4100 m), with
mean age 20.03 � 3.07, were studied in our laboratory at 3510 m.
Finger oximetry was performed on sitting subjects during 15 minutes, of
which the last 5 minutes were used for analysis. A computer hook-up via
serial port from a BCI portable oximeter plotted and stored the oxygen
saturation and pulse every 5 seconds. Subjects were prevented from observing
the results, in order to avoid them changing their breathing pattern. In
6 of these subjects, measurements were continuous while the door locks
of the Hyperoxic\Hypoxic Adaptation Chamber (HHAC) were closed and the
partial inspired oxygen tension (PIO2) was raised from breathing ambient
air (AA) (PIO2 = 94 mmHg) to simulated sea level (SSL) values (PIO2 = 150
mmHg) without changing the barometric pressure (PB = 494 mmHg) at 23°C.
After breathing 15 minutes in the chamber, oximetry recordings during the
last 5 minutes were used for analysis.
Maximum (max) and minimum (min) values, mean, standard deviation and
frequency distribution of oxygen saturation (SAT) as well as pulse of all
the subjects were calculated and plotted. Although individual variations
are evident, using finger oximetry, the average results show that significant
variations in oxygen saturation and pulse of native residents of high altitude
are diminished when exposed to simulated sea level in the HHAC.
INTRODUCTION
Pulse oximeters have been described as "remarkable among monitors in that it involves no calibration, negligible time lag and infrequent false negative data and requires no routine maintenance" (1). However, those that have been at high altitude and used an oximeter, have found that the readings had large variations, to the point that malfunction of the apparatus was even considered. Nocturnal desaturation at high altitude has been studied (2, 3, 4), but daytime oscillations have not been reported. Also, during flight, large saturation variations between individuals have been observed (5).
In order to study the saturation variations in natives living at 3510 m, measurements breathing ambient air and simulated sea level in the hyperoxic/hypoxic adaptation chamber (HHAC) were recorded. This allowed evaluation of the same pulse oximeter without long time spans as well as wheter the same oximeter used under sea level partial inspired oxygen tension (PIO2) would perform as expected.
METHODS
A BCI portable oximeter was hooked up via serial port to a computer
which plotted saturation and pulse every 5 seconds. A finger probe was
placed in the right index finger. The subjects were sitting inside the
hyperoxic\hypoxic adaptation chamber. The doors of the door lock were open
and each subject was breathing ambient air at 3510 m, with barometric pressure
of 495 mmHg and partial inspired oxygen tension (PIO2) = 94 mmHg. Following
15 minutes of continuous recording, the doors of the lock were closed and
the oxygen percentage in the chamber was raised to 32% that corresponds
to a sea level PIO2 of 150 mmHg at the same barometric pressure. Air recirculated
through a soda lime absorber, prevented CO2 rise. The temperature was kept
constant at 23° Centigrade.
Twenty natives of high altitude (Age mean = 20.03 ± 3.07)
were studied breathing ambient air and 6 of those also under simulated
sea level conditions. Average and standard deviations as well as coefficient
of variance were calculated in both groups using a Qpro spreadsheet.
RESULTS:
The results are shown in table 1. Saturation averages of each group
varied from 95.4 % (maximum) to 89.0 % (minimum) during ambient air
conditions and changed to 98.4 % (maximum) to 97.0 % (minimum) during simulated
sea level conditions. Similarly, pulse variations can also be observed.
A frequency distribution (C) of the average of the last 5 minutes of observations
in the 6 patients breathing ambient air (A) and during hyperoxia in the
HHAC (B) is shown in fig. 1.
| MAX | MIN | MEAN ± SD | COEFF OF VARIATION | |
| AA SAT in % | 95.4 | 89.0 | 92.0 ± 1.28 | 1.40 |
| SSL SAT in % | 98.4 | 97.0 | 97.7 ± 0.34 | 0.35 |
| AA PULSE in bpm | 72.4 | 60.2 | 65.2 ± 2.69 | 4.16 |
| SSL PULSE in bpm | 63.8 | 53.6 | 59.0 ± 2.27 | 3.79 |
DISCUSSION:
The troublesome reading of saturation in a pulse oximeter at high altitude
is evident and usually not an apparatus malfunction. Several reports on
the response of oximeters under low perfusion thresholds, profound hypoxia,
anemia, show manufacturer variations of response in pulse oximeters (6
,7, 8). However the variation at high altitude did not seem to be reported.
The evident decrease of the coefficient of variability when the subjects
were in the HHAC, shows that at sea level (acute exposure) the oximeter
performs as expected and therefor malfunction can be ruled out.
As expected, the reference values of pulse oximetry at high altitude,
are conflicting because not only are age, sex, race, different physiological
states such as sleep and health status involved, but also the different
altitudes. At 2640 m the mean saturation in children is reported at 91.1%
during sleep and 93.3 % in daytime activity (9).
Some variations of pulse oximetry in newborns at 1610 m is reported and questioned for ‘what is normal?” (10). Although that study is only at 1620 m, the variations in measurement of saturation may have given rise to the question.
The reports that measurement of saturation can aid in the diagnosis of pulmonary illness in children at high altitude (11, 12, 13) are interesting but the variations we report should be taken into consideration. Quick measurements of saturation can be deceiving. In our long experience with high altitude, the resting steady state is difficult to achieve and a minimal psychological unrest will affect it considerably. Changes in the respiratory frequency and tidal volume, as in deep inspiration (and even talking) where a 99% saturation at 3600 m can be reached, make it difficult to maintain a steady state ventilation.
It is also important to consider that at 3510 m, the average saturation being 92 %, one deep breath can increase the saturation to 99 % in some subjects. The shape of the oxygen dissociation curve should also be taken into account. Saturation fluctuations in the steeper portion are obviously greater, however, ventilatory irregularities which are typical of high altitude are the main cause. Furthermore, apneas may not only be present during sleep, but also while being awake. This implies constant change in blood pH, and CO2 elimination, with frequent shifts in the p50 of the oxygen dissociation curve. Some authors report that at high altitude the in vivo p50 is shifted to the left (14, 15). Others say that it is shifted to the right (16, 17) and some that it remains the same (18). Still others claim that measurements below 14,000 ft are left shifted and those above 14,000 feet right shifted (19). Saturation variations may be a reason why reports of in vivo p50 have been conflicting.
When blood gases are about to be drawn, a hyper-ventilation or a hypoventilation at this altitude, can cause great variations. In patients with chronic mountain sickness that are suffering the triple hypoxia syndrome (20), these fluctuations are in the steepest part of the curve.
Our bottom line advice is to be careful while measuring oxyhemoglobin saturation with a pulse oximeter at high altitude.
Fig.1 Frequency distribution of the last 5 minutes of six high altitude natives at 3510 m (Pb = 495 mmHg) breathing ambient air PIO2 = 95 mmHg (A) and during hyperoxia PIO2 = 150 mmHg in the HAAC (B). The average of both groups {n=20 for ambient air in blue and n=6 for hyperoxia in red} are plotted in graph (C).
BIBLIOGRAPHY
1. Severinghaus JW: Review Article: Recent developments in pulse
oximetry. Anesthesiology 76 (6)(Jun.): 1018-1038, 1992
2. Fujimoto K, Matsuzawa Y, Hirai K, Yagi H, Fukishima M, Shibamoto
T, Koyama S, Levine BD, Kobayashi T: Irregular nocturnal breathing patterns
at high altitude in subjects susceptible to high-altitude pulmonary edema
(HAPE): A preliminary study. Aviat Space Environ Med 60: 786-791, 1989
3. Anholm JD, Powles AC, Downey R, Houston CS, Sutton JR, Bonnet
MH, Cymerman A: Operation Everest II: Arterial oxygen saturation and sleep
at extreme simulated altitude. Am Rev Respir Dis 145(4): 817-826, 1992
4. West JB, Peters Jr RM, Aksnes G, Maret KH, Milledge JS, Schoene
RB: Nocturnal periodic breathing at altitudes of 6300 and 8050 meters.
J Appl Physiol 61(1): 280-287, 1986
5. Bendrick GA, Nicolas DK, Krause BA, Castillo CY: Inflight
oxygen saturation decrements in aeromedical evacuation patients. Aviat
Space Environ Med 66(1): 40-4, 1995
6. Severinghaus JW, Naifeh KH, Koh SO: Errors in 14 pulse oximeters
during profound hypoxia. J Clin Monit 5: 72-81, 1989
7. Falconer RJ, Robinson BJ: Comparison of pulse oximeters: accuracy
at low arterial pressure in volunteers. Br J Anaesth 65(4): 552-557, 1990
8. Hannhart B, Haberer JP, Saunier C, Laxenaire MC: Accuracy
and precision of fourteen pulse oximeters. Eur Respir J 4(1): 115-119,
1991
9. Lozano J, Duque O, Buitrago T, Behaine S: Pulse oximetry reference
values at high altitude. Arch Dis Child 67(3): 299-301, 1992
10. Thilo E, Park-Moore B, Berman E, Carson B: Oxygen saturation
by pulse oximety in healthy infants at an altitude of 1610 m (5280 ft).
What is normal? Am J Dis Child 145(10): 1137-40, 1991 11. Nicholas R, Yaron
M, J R: Oxygen saturation in children living at moderate altitude. J Am
board Fam Pract 6(5): 452-6, 1993
12. Beebe S, Heery L, Magarian S, Culberson J: Pulse oximetry
at moderate altitude. Healthy children win upeer respiratory infection.
Clin Pediatr (Phila) 33(6): 329-32, 1994
13. Reuland D, Steinhoff M, Gilman R, Bara M, Olivares E, Jabra
A, Finkelstein D: Prevalence and prediction of hypoxemia in children with
respiratory infections in the Peruvian Andes. J Pediatr 119(6): 900-6,
1991
14. Winslow RM, Samaja M, West JB: Red cell function at extreme
altitude on Mt. Everest. J Appl Physiol 56(1): 109-116, 1984
15. Aberman A, Hew E: Clarification of the effect of changes
in P50 on oxygen transport. Acute Care 11(3-4): 216-21, 1985
16. Mairbaurl H, Schobersberger W, Oelz O, B�rtsch P, Eckardt
KU, Bauer C: Unchanged in vivo P50 at high altitude despite decreased erythrocyte
age and elevated 2,3 diphosphoglycerate. J Appl Physiol 68(3): 1186-1194,
1990
17. Mairbaurl H, Oelz O, Bartsch P: Interactions between Hb,
Mg, DPG, ATP, and CL determine the change in Hb-O2 affinity at high altitude.
J Appl Physiol 74(1): 40-8, 1993
18. Winslow RM, Monge CC, Statham NJ, Gibson CG, Charache S,
Whittembury J, Moran O, Bergen RL: Variability of oxygen affinity of blood:
Human subjects native to high altitude. J Appl Physiol 51(6): 1411-1416,
1981
19. Keys A, Hall FG, Barron ES: The position of the oxygen dissociation
curve of human blood at high altitude. Am J Physiol 115: 292-307, 1936
20. Zubieta-Castillo G, Zubieta-Calleja G: Triple
hypoxia syndrome. Acta Andina 5(1): 15-18, 1996.